
![]() Quantum
puzzles. Of the various quantum puzzles, the most basic is probably
wave-particle duality. The atom itself is, however, the most important, puzzling
consequence for the ordinary world. The traditional way of summing up what
is most puzzling about quantum mechanics is the Heisenberg uncertainty principle,
but recently the most discussed is called “Bell’s inequality.” All of them
are described here as a way of introducing quantum mechanics as it is currently
understood, and after explaining the spatiomaterialist theory of quantum matter,
I will show how they can be solved.
Quantum
puzzles. Of the various quantum puzzles, the most basic is probably
wave-particle duality. The atom itself is, however, the most important, puzzling
consequence for the ordinary world. The traditional way of summing up what
is most puzzling about quantum mechanics is the Heisenberg uncertainty principle,
but recently the most discussed is called “Bell’s inequality.” All of them
are described here as a way of introducing quantum mechanics as it is currently
understood, and after explaining the spatiomaterialist theory of quantum matter,
I will show how they can be solved.
 Wave-particle
duality. According to Bohr, the basic puzzle of quantum mechanics is
the dual nature of the basic entities it describes. They all appear to be
like both particles and like waves. What classical physics took to be waves
turn out to have a particle-like nature as well, and what classical physics
took to be particles turn out to have a wave-like nature as well. Bohr thought
that both appearances of the underlying reality are due in part to our measuring
apparatus and the classical expectations on which they are constructed. But
wave-like and particle-like natures are apparently incompatible, and since
both of these classical conceptions of reality are needed to make all of the
possible predictions, he called the basic puzzle of quantum mechanics “complementarity.”
Bohr was the originator of the so-called "Copenhagen interpretation"
of quantum mechanics, which holds that the reality behind these complementary
phenomena is incomprehensible to us.
Wave-particle
duality. According to Bohr, the basic puzzle of quantum mechanics is
the dual nature of the basic entities it describes. They all appear to be
like both particles and like waves. What classical physics took to be waves
turn out to have a particle-like nature as well, and what classical physics
took to be particles turn out to have a wave-like nature as well. Bohr thought
that both appearances of the underlying reality are due in part to our measuring
apparatus and the classical expectations on which they are constructed. But
wave-like and particle-like natures are apparently incompatible, and since
both of these classical conceptions of reality are needed to make all of the
possible predictions, he called the basic puzzle of quantum mechanics “complementarity.”
Bohr was the originator of the so-called "Copenhagen interpretation"
of quantum mechanics, which holds that the reality behind these complementary
phenomena is incomprehensible to us.
The particle-like nature of electromagnetic waves. Light has long since been thought to have wave-like nature in classical physics. Early in the nineteenth century, Thomas Young showed that light passing through two narrow, closely spaced holes (or slits) produces a pattern of light and dark lines on the screen that finally intercepts them, and he explained it by the wavelike nature of light. The places on the screen where the waves emerging from each slit interfere constructively are bright, while the places where they interfere destructively are dark. When one slit is blocked, the interference pattern disappears.
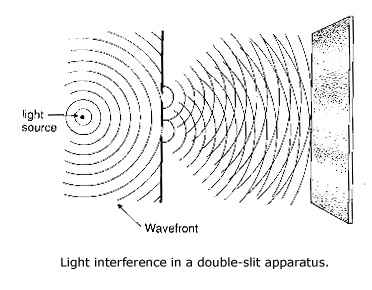
Diffraction phenomena also indicate the wave like nature of
light. Light rays passing through a hole that approximates its wavelength
will be spread out as it leaves the hole, with the range of the spread varying
inversely with the size of the hole. When the hole is large, the light hitting
the distant screen is like a point, but when the hole is small, the diffraction
is great.
Moreover, as we have seen, light had been explained by Maxwell
as the wavelike coupling of the electric and magnetic forces described by
his equations. Indeed, it enabled him to predict the velocity of light.
The particle-like nature of light was the first of the discoveries
that eventually culminated in quantum mechanics. Instead of propagating like
a wave in an elastic medium, as the classical model assumed, it became clear
that light is actually made up of distinct particles, which are now called
“photons”. This particle-like nature means that the energy and momentum carried
by light do not combine continuously, as they do in ordinary waves, but come
in separate units, called “quanta”. The size of the quantum of light is now
represented by Planck’s constant, h, which is part of every new equation
used in quantum mechanics. It appears in the new equations for the energy
and momentum of light. The energy, E, is given by E = hf
(where E is energy, f
is frequency), and the momentum, p, is given by p = h/l (where p is momentum, and l is the wavelength).
Max Planck first discovered the particle-like nature of light
in 1900, though he did not fully understand what he was on to. He discovered
the constant named after him by tinkering with a classical equation for calculating
the amount of energy given off at each frequency in so-called blackbody radiation,
that is, a hot body in which no frequency of light should be favored. (It
is best approximated by a box with mirrored interior walls in which light
of all possible wavelengths for a box with certain temperature are being reflected
back and forth.) The classical equation assumed that the frequencies of light
being given off varied continuously from the lowest to the highest, with the
peak intensity depending on the temperature. That assumption worked well enough
for the low frequencies, but at high frequencies, it led to the conclusion
that the total energy given off should be infinite. This absurdity was called
the “ultraviolet catastrophe.” Planck discovered a formula that avoided the
catastrophe and predicted the total quantity of energy given off at each frequency
by introducing a constant, h, into the formula which restricted the
frequencies of light. That is the source of the equation for the energy of
light: E = hf. (Though its meaning is still obscure, it can,
perhaps, be seen as requiring the photons to differ from one another by that
constant amount.)
Albert Einstein made it clearer that what Planck had discovered
was the particle-like nature of light by using Planck’s constant is his own
explanation of the photoelectric effect (in 1905, the same year that he published
his special theory of relativity). It had been known that light being intercepted
by material objects could release electrons from the material objects, but
it was found that the release of electrons did not depend on the total energy
of the light waves (the intensity of the light), as one would expect on the
wave hypothesis. It depends on the frequency of the light. Below a certain
frequency, no electrons are released, regardless how intense the light may
be at that frequency. Whereas light with a higher frequency would release
electrons even though the intensity was much less. Einstein showed that the
release of the electrons depended on the absorption of single photons, each
of whose energy depended on Planck’s constant: E = hf.
Much later (in 1923), Arthur Compton showed that photons also have a momentum like particles. He shot high energy photons (x rays) at electrons and used arguments based on the conservation of momentum and energy to predict correctly the amount by which their energies would be changed by such scattering.
The particle-like nature
of the light does not change its wave-like properties. Indeed, it turns out
that interference effects still occur when light is sent through the two-slit
apparatus one photon at a time. Over time, they still accumulate in fringes
on the distant wall.
The wave-like nature of
particles with rest mass. Material objects are understood in
classical physics as having definite locations in space at each moment and
to follow definite trajectories as they move from one place to another. But
the behavior of objects with rest mass on the smallest scale is peculiar in
the opposite way from photons, according to quantum theory. Just as light
waves have a particle-like nature, so material objects have a wave-like nature.
The wave-like nature of particles with rest mass was predicted
in 1923 by de Broglie. What Einstein’s special theory of relativity implies
about the relativistic increase in mass leads to the conclusion that the energy
of a photon is equal to the product of its momentum and the velocity of light,
or E = pc. Since the velocity of light is equal to the product
of the frequency and wavelength, or c = fl, it follows that the momentum
of a photon is p = h/l. De
Broglie went on to suggest that the same relationship holds of particles with
kinetic energy. He argued that particles, such as electrons, protons and material
objects with mass generally would also have a wave length that varied inversely
with their momentum in the same way.
Interference and diffraction phenomena were the kind of empirical
evidence that was taken as showing that light has a wave-like nature, and
soon after de Broglie’s prediction, it was shown that the electrons forced
to pass through very small holes do exhibit diffraction, that is, the smaller
the hole, the more they spread out. Eventually, even interference phenomena
were demonstrated with electrons. When electrons moving at a certain velocity
are projected through narrow, closely spaced, parallel slits at a screen (where
the distance between the slits approximates their de Broglie wave length),
they also form an interference pattern on the far wall, as if they were waves.
Even when the electrons were sent one at a time, they tended to land on the
distant screen only along certain fringes, leaving lines between them without
any hits. Thus, each particle is like a wave. The same has been show to hold
for neutrons, though in the case of ordinary sized objects, the wavelengths
are so small that interference effects are undetectable.
 The
structure of the hydrogen atom. The laws of quantum mechanics were
discovered mainly by attempting to explain the structure of the hydrogen atom.
It had been established by Ernest Rutherford that the atom is composed of
a massive, positively charged nucleus surrounded by far less massive electrons,
and Niels Bohr hoped to explain the chemical properties of atoms by the nature
of the interactions between the electrons and the nucleus. It was clear that
atoms could not be explained in classical terms on the model of the solar
system, since according to Maxwell’s equations, the orbital motion of an electron
would generate (as the acceleration of a negatively charged particle) an electromagnetic
wave which would drain its energy until the electron was located at rest with
the nucleus. In fact, atoms with electrons located around it are quite stable,
and when such atoms are excited (by supplying energy to them), they give off
electromagnetic radiation at a certain set of distinctive frequencies.
The
structure of the hydrogen atom. The laws of quantum mechanics were
discovered mainly by attempting to explain the structure of the hydrogen atom.
It had been established by Ernest Rutherford that the atom is composed of
a massive, positively charged nucleus surrounded by far less massive electrons,
and Niels Bohr hoped to explain the chemical properties of atoms by the nature
of the interactions between the electrons and the nucleus. It was clear that
atoms could not be explained in classical terms on the model of the solar
system, since according to Maxwell’s equations, the orbital motion of an electron
would generate (as the acceleration of a negatively charged particle) an electromagnetic
wave which would drain its energy until the electron was located at rest with
the nucleus. In fact, atoms with electrons located around it are quite stable,
and when such atoms are excited (by supplying energy to them), they give off
electromagnetic radiation at a certain set of distinctive frequencies.
Bohr explained the frequencies of the spectrum of hydrogen atoms
(in 1913) by assuming that electrons can have only certain orbits, each characterized
by an energy level that corresponds to the total energy of an electron with
kinetic energy in a force field with potential energy imposed by the nucleus.
(The total quantity of energy is negative, because the kinetic energy of the
particle is not great enough to replace all the negative potential energy
that would be required to free it, and according to our assumption about the
nature of potential energy, the negative sign for potential energy indicates
that the nucleus and electron have less rest mass.) The energies of the possible
orbits were determined as a function of Planck’s constant, and a number was
assigned to each possible orbit, starting with the lowest energy orbit and
counting upwards (n = 1, 2, 3, . . ). Bohr showed that the spectral
lines of the hydrogen atom could be explained by the differences in the energies
of these permitted electron orbits.
The basic puzzle of quantum mechanics is the structure of the atom itself, that is, what is going on that only certain energy levels are possible for electrons bound to a nucleus by electromagnetic forces.
Given the structure of the atom, however, there is another
problem, for it does not seem possible that electrons could be jumping from
one orbital to another. When a photon is absorbed or emitted by an atom, an
electron changes from one permitted orbital to another (so that the atom changes
from one energy state to another). But the photon has a particle-like nature,
and the particle seems to change its position and motion in an instantaneous,
step-like change, that is, without accelerating nor even moving continuously
from one state to the next. It hard to see how the electron’s change of orbital
can be explained as the motion of a material object, since a material object
can change location only by moving across space continuously as time passes
time.
Another puzzle has to do with the timing of the emission of
photons. When an atom or molecule is in an energy state that can decay into
a lower energy state, it is not possible, even in principle, to say exactly
when it will decay. The timing can be assigned a probability, but the theory
has nothing to explain why it happens at one moment rather than another within
that range.
Electron jumps also seem to be involved in the phenomenon of
tunneling. “Tunneling” refers to situations in which electrons seem to jump
across barriers imposed by force fields. On classical principles, crossing
such a force field would require more energy than the electron has. Nevertheless,
some electrons do jump across. Only a few electrons do so, and there is no
way to predict which ones will jump. But it is so regular that this phenomenon
is used as a kind of microscope for mapping the surfaces of material objects.
Erwin Schrödinger thought that it would be possible to avoid
these puzzles about electron jumps and explain everything deterministically
by following up on de Broglie’s suggestion and explaining the behavior of
the electron in an atom as a wave. Using the model of the classical equation
for waves and taking the electron wave to be in a potential field, Schrödinger
presented an equation in 1925 that explained the energy levels of the permitted
orbitals of electrons in the force field imposed by the nucleus of the hydrogen
atom. The time-independent Schrödinger equation (with the temporal changes
factored out so that it represents only the spatial structure of the wave)
portrays the electron bound to the nucleus of the hydrogen atom as a standing
wave, like a plucked string on a guitar. This made it possible for Schrödinger
to explain the numbers that Bohr had assigned to the permitted orbitals of
electrons as the energy states in which the electron could be such a stable,
standing wave. The lowest energy level corresponds to the string with no nodes
(that is, half the wave length for its energy), the next one to a string with
one node, and so on. The problem of quantum jumps seemed to be solved, because
the transitions between such energy states of atoms were explained as smooth
and continuous transitions of waves.
Schrödinger believed that his wavefunction showed that electrons
were not particles at all, but could be explained purely as waves in an electromagnetic
field. This did not explain why electrons appear to be particles, for example,
how they leave vapor trails in a Wilson cloud chamber or interact at a certain
point on the distant wall in the two-slit interference experiment. But it
is possible to explain why electrons seem to have a determinate location by
holding that they are a "superposition" of waves with slightly different
wavelengths, because in regions where such wave interfere constructively,
they clump together in what are called “wave packets.” Since the locations
where such a set of waves interfere constructively have more or less precise
locations in space and seem to move through the space occupied by the waves,
the Schrödinger wavefunction could explain the appearance that electrons move
like particles. (This was not a fully adequate explanation, however, because
such wave packets also tend to disperse over time, and yet electrons actually
turn up later at definite locations.)
However, it was not possible to interpret the Schrödinger wavefunction
as the description of a classical wave. One problem was that it contained
complex numbers. There is no way to measure quantities multiplied by the square
root of minus one, and yet those complex numbers are essential to the wavefunction,
since they describe the phases of the waves that are superimposed in the quantum
system and, thereby, determine the interference phenomena.
Furthermore, the Schrödinger wavefunction described a wave
in a space that can have more than three dimensions (or what is called “configuration
space). When more than one particle is involved, the space occupied by the
wave described by Schrödinger’s wavefunction has three times as many dimensions
as there are particles. There is no obvious
way to relate such an equations to the actual three dimensional world.
What is now the orthodox interpretation of the Schrödinger wavefunction was first proposed by Max Born in 1926. He took the square of the (time-independent) wavefunction in some region of configuration space to be a measure of the probability of finding that the particle located in that region of configuration space (thereby predicting a measurable property, such as location, momentum or kinetic energy). The predictions are confirmed by measurement.
Since the predictions are merely probabilistic predictions,
however, Born took the Schrödinger wavefunction to be a representation, not
of the world itself, but of what we can know about it. This avoided the problems
of quantum jumps and wave packets that spread out, because what really happens
is not knowable. And insofar as it is taken realistically, it implies that
what happens is not fully determined by the state that precedes it.
 Heisenberg
uncertainty principle. An entirely different mathematical representation
of these same quantum phenomena was developed by Werner Heisenberg. His “matrix
mechanics” is basically an algorithm for making predictions of measurements
without any attempt to explain what is going on beneath the observable surface.
Though Schrödinger showed that Heisenberg’s matrix mechanics and his own wavefunction
are mathematically equivalent, matrix mechanics makes the limitations on what
can be known about the classical properties of the entities described by quantum
mechanics clear. In arguing against Schrödinger, he defended what has come
to be known as the Heisenberg uncertainty principle.
Heisenberg
uncertainty principle. An entirely different mathematical representation
of these same quantum phenomena was developed by Werner Heisenberg. His “matrix
mechanics” is basically an algorithm for making predictions of measurements
without any attempt to explain what is going on beneath the observable surface.
Though Schrödinger showed that Heisenberg’s matrix mechanics and his own wavefunction
are mathematically equivalent, matrix mechanics makes the limitations on what
can be known about the classical properties of the entities described by quantum
mechanics clear. In arguing against Schrödinger, he defended what has come
to be known as the Heisenberg uncertainty principle.
In matrix mechanics, there are pairs of variables called “complementary”
or “conjugate” variables, because the measurement of one affects the measurement
of the other. That is, the results of measuring one variable and then the
other would be different if they were measured in the opposite order. The
position and momentum of an electron are complementary variables, meaning
that the position and momentum of an electron cannot both be measured with
arbitrarily high precision But the more precise one measurement is, the less
precise the other is. Using Born’s probabilistic interpretation of the wavefunction
to express the “uncertainties” in such measurement, Heisenberg derived a general
principle about complementary variables: the product of the uncertainty about
the position and the uncertainty about the momentum cannot be less than Planck’s
constant divided by four pi.
Heisenberg’s uncertainty principle holds in a parallel way
for other conjugate variables, such as energy and time, angular momentum and
orientation, and cycle and phase. In each case, one variable is more particle-like
and the other is more wave-like, and thus, the variables are said to be complementary.
Heisenberg apparently took his uncertainty principle to be
a basic postulate from which all of quantum mechanics could be developed.
He rejected talk about the wave-particle duality and took a purely instrumentalist
approach which simply denied that there is any aspect of the world that is
not described by his matrix mechanics (or by their equivalents in using the
Schrödinger wavefunction).
The equivalence of Heisenberg’s matrix mechanics and Schrödinger’s
equation means that the Heisenberg uncertainty principle can be derived in
a similar way from Schrödinger’s equation.
The solution of Schrödinger’s equation for a given situation
yields a wavefunction, which is a complete description of the quantum system.
But in order to predict a measurable property, it is necessary to apply an
appropriate mathematical operator to the wavefunction. The operator yields
an “expectation value” for that property, which may be a precise value or
an average value.
But some pairs of operators are not commutable, such as the
position and momentum of a particle. Though it is often possible to make precise
predictions of these properties, the prediction of one makes it impossible
to predict the other. That is, when one property is predicted by one operator,
the mathematical operation changes the wavefunction and so the prediction
made for the other property is not the same as it would have been if the second
property had been predicted first. Since the order in which the operators
are applied to the wavefunction makes a difference in what they predict, it
is impossible to predict both properties at once. Thus, the conjugate variables
to which Heisenberg’s uncertainty applies turn out to be the pairs of properties
predicted by non-commutable operators.
When the operator yields an expectation value that is just the average result for an entire series of experiments, it can often be represented as a superposition of different wavefunctions for each of which the operator gives an expectation value. When the measurement is made and one of them turns out to be true, the wavefunction is said to “collapse,” because the system turns out to have one or another of precise predicted outcomes. This is called the “collapse of the wavefunction,” because it is assumed that prior to the measurement, what actually existed was a superposition of different wavefunctions.
This interpretation of the measurement of a quantum system exacerbates the problem, for the superposed states of the system can evolve in radically different ways. In the most famous example, a cat is locked in a box with a devise triggered by an unpredictable beta decay that will, with 50% probability, release a poison that kills the cat within a certain period of time. But until someone looks to see what has happened, there is a superposition of the two states, one with a dead cat and another with a living cat, and reality only resolves itself into one or the other possibility at the moment someone looks. This implausible implication of measurement being the collapse of the wavefunction is called the problem of "Schrödinger’s cat."
The Heisenberg uncertainty principle is, perhaps, the most general
statement of the puzzles of quantum mechanics, and a genuine ontological explanation
of quantum mechanics, if there is one, should reveal the source of this limitation
on our knowledge.
 Bell
correlations. Recently, attention has focused on a final quantum mystery,
called “Bell’s Theorem” or “Bell’s Inequality.”[1]
John Bell showed that quantum mechanics entails, in certain circumstances,
a statistical correlation between events occurring at a distance that seems
to be possible only if the events have effects on one another that travel
faster than the velocity of light. It holds for interactions in which particles
move away from one another in opposite directions with opposite orientations
of a “spin”.
Bell
correlations. Recently, attention has focused on a final quantum mystery,
called “Bell’s Theorem” or “Bell’s Inequality.”[1]
John Bell showed that quantum mechanics entails, in certain circumstances,
a statistical correlation between events occurring at a distance that seems
to be possible only if the events have effects on one another that travel
faster than the velocity of light. It holds for interactions in which particles
move away from one another in opposite directions with opposite orientations
of a “spin”.
Spin. Spin is a quantum property that was first recognized with the discovery of quantum field theory. The Schrödinger wavefunction is the law of non-relativistic quantum mechanics, and a more complete law was discovered by Paul Dirac when he combined the Schrödinger wavefunction with Einstein’s special theory of relativity, that is, taking the relationship it describes between space and time into account.
There was an asymmetry between the time-dependent and time–independent wavefunctions derived by solving Schrödinger equation. The time-independent wavefunction, describing the spatial aspects of the standing wave, is a second order differential equation, whereas the time-dependent wavefunction, describing how the quantum system unfolds in time, is a first order differential equation. Dirac derived a time-dependent wavefunction that was a second order differential equation, making time and space symmetrical, as they are in the special theory of relativity.
It is puzzling just what makes Dirac's derivation work, but
it involved several profound discoveries.
Dirac discovered that there are twice as many solutions for
the wavefunctions than had been thought, half of them corresponding to negative
energy. This was the discovery of antimatter, such as, for example, the positively
charged electron as the negative partner of the negatively charged electron,
called the "positron."
Dirac discovered that quantum particles have another property,
called “spin,” which was a new quantum
number that was needed for wavefunctions to describe fully any quantum situation.
That is, spin is a new quantum number (namely, s) needed to describe
the atom (along with Bohr’s numbers for the energy states of atoms (n),
a number for the orbital angular momentum of the electron (i), and
a number for its magnetic moment (m)).
It is believed that the intrinsic spin of an electron has little
to do with a spinning electrical charge. The spin of a particle is defined
operationally as the strength of the magnetic force that results when a magnetic
field is imposed on the particle. Particles, such as the electron, that have
½ spin (called “fermions”) have one of only two possible magnetic moments
(positive and negative). Since there is no way for them not to have a magnetic
moment, it is hard to see how they could be a classical material object with
a charge that is somehow actually spinning.
Bell’s Inequality. John Bell discovered a curious consequence of quantum mechanics involving spin. The spin of a particle (either a rest mass or a photon, which has a spin of 1) would seem to a property that the particle carries with it, but a prediction made on this assumption contradicts quantum mechanics. And it seems to have been disproved empirically. This suggest that spin is a property that depends, not on the particle itself, but on what happens elsewhere in a much more inclusive system involving both particles.
The system is one in which two objects are generated in a way that requires them to have opposite orientations of spin, and they move away from one another in opposite directions. Since space is three dimensional, the spin of a particle can be measured from three different, mutually perpendicular directions. If one particles is measured as having as having spin, say, up, in some direction, then the other particle will never turn out to have anything but the opposite, down, orientation of spin when it is measured in the same direction. This holds regardless which of the three independent directions in space the magnetic field is oriented, and quantum mechanics does not permit one to infer from its spin in one direction what its spin in any other direction is. Thus, if spin is a property that the particles already have when they part from one another, the outcome of measuring the spin of the particles that moved off one way from their creation from one direction should not enable us to predict the spin of the other particle when measured from a different direction. Bell showed that, on this assumption, a certain inequality must hold about the frequency with which measurements of spin in one particle in one direction would correlate with measurements of the spin of the other particle in one of the other directions.
However, quantum theory predicts and experiments have confirmed
that this inequality will be violated. When two objects are generated in this
way, and the spin orientation of one of these objects is measured in one direction,
it is possible to predict the outcome of a measurement of the spin orientation
(up or down) of the other object in an independent direction of three dimensional
space more often than the Bell inequality allows. It is not a reliable prediction
in any particular case, but statistically it is more frequent than would be
possible, if the spin orientations of both objects were already determined
when they parted and they were simply carried away with them, as the principle
of local action would require.
Though the two measurements can be made as far apart in space
as one likes, it seems that the only way the measurements could be correlated
is if the measurement of one object were somehow affecting the state of the
other. And since the two measurements can be made to occur as near to one
another in time as one likes, there are instances of this phenomenon in which
such an effect could hold only if something travels between them faster than
the velocity of light. This puzzling correlation is not only a consequence
of quantum theory, but has also been confirmed experimentally, and thus, it
seems that we must give up the principle of local action. But it seems to
violate the principle of local action.
The puzzles of quantum mechanics have to do with understanding
what in the world corresponds to the Schrödinger equation. The “Copenhagen
interpretation” of quantum mechanics, developed by Bohr, is the received view.
It simply denies that it is possible to describe the nature of what exists
except by applying the classical conceptions of particles or wave, which if
not strictly speaking incompatible, are, at best, complementary. Defenders
of the Copenhagen interpretation see the puzzles of quantum mechanics as deriving
from its departures from classical physics, as if classical physics were based
on intuitions ( or a form of imagination) that is anthropocentric and, thus,
merely subjective. And some go on to insist that the uncertainty is a real
indeterminism about what happens in the world.
The chief opponent of this view was Einstein. He was resisting
the reification of quantum uncertainty as indeterminism when he claimed, “God
does not play dice with the universe.” A view of the world as being constituted
by substances of some kind is what kept Einstein from accepting quantum mechanics
as the complete description of what exists. His acceptance of spacetime as
a substance made him most sympathetic to Spinoza, for Spinoza believed that
the world is a single substance. But what seems to have kept Einstein from
admitting that such a substance could have indeterminism as a basic property
were his ontological instincts.
In what follows, I will elaborate the the assumptions of spatiomaterialism
in a way that explains ontologically why quantum mechanics is true. It is,
as I have warned, more speculative than the rest of the argument of ontological
philosophy. But it may suggest the power of an ontological approach and vindicate
Einstein’s view of the nature of the world in at least one respect.